Ex vivo identification of specific gene vector capsids for tumor-targeted gene therapy with adeno-associated viral vectors
People involved: Jakob Körbelin & Merrit Rothe in collaboration with Martin Trepel & Bruno Märkl (Augsburg)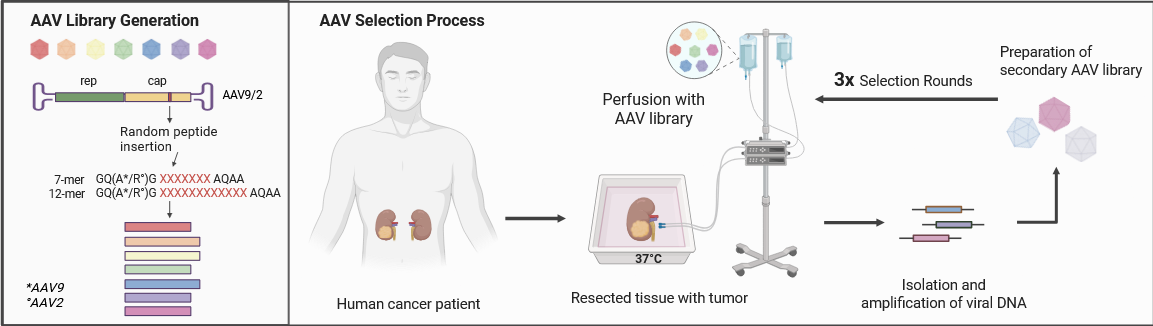 Created in BioRender. Rothe, M. (2025) https://BioRender.com/i5kffdz
Created in BioRender. Rothe, M. (2025) https://BioRender.com/i5kffdz
Background
The treatment of cancer has been a major field of research for decades but it remains one of the biggest clinical challenges. In 2021, cancer lead to approximately 22% of all deaths across the European Union. The number of cancer patients increases every year, reinforcing the urgent need for more effective and targeted therapies. Cancer gene therapy offers promising possibilities for supplementing conventional cancer therapies. Our lab is specialized on the development of Adeno-associated virus (AAV) vectors, which benefit from high transgene expression levels and the ability to transduce a variety of mammalian cell types. In preclinical studies, recombinant AAVs equipped with a broad range of promoters and transgenes successfully reduced tumor growth and burden in mice. In this project, we aim to develop AAV vectors that specifically target solid human tumors or tumor endothelial cells and that can be used in cytotoxic or immune modulatory anti-tumor gene therapies.
Scientific Approach
To alter the natural tropism of the AAVs, we modify the AAV capsid by incorporating random peptide libraries. These peptides are displayed on prominent protrusions at the 3-fold axis of symmetry on the serotypes AAV9 & 2 (VP1 positions A589 and R588, respectively). We established a novel ex vivo AAV selection process for AAV libraries in human organs. To this end, we use resected human colon and kidney tissue from colon and kidney cancer patients directly after surgery and perfuse the tissue with AAV libraries. The entire selection process involves three selection rounds with pre-defined numbers of perfused organs per round and with organs from different sexes. Following perfusion, the DNA of the enriched AAV variants is isolated from healthy and tumor tissue and a secondary AAV library is generated after each selection round from the viral DNA found in the tumor tissue. The diversity of the initial AAV library is being reduced in each round and assisted by NGS analysis, we can determine the most strongly enriched and most tumor-specific AAV variants. This Project is funded by the German Research Foundation (DFG) under the project number 523 830 300.
