Molecular Pathogenesis of Pulmonary Vascular Diseases
PI: Dr. Jan K. Hennigs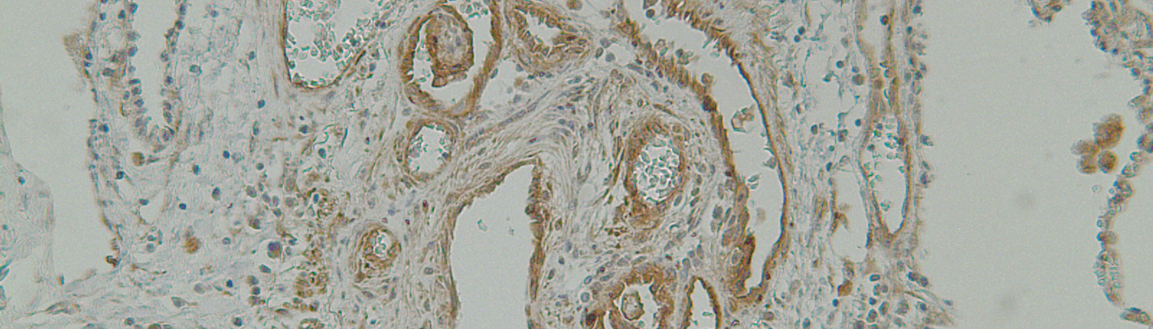
Background
Pulmonary vascular diseases (PVD), and pulmonary arterial (PA) hypertension (PAH) in particular, are life-threatening diseases. These conditions are characterized by pathological structural changes to the lung vessels called remodeling.
Lung vessel "remodeling" is associated with progressive narrowing of the vessel lumen causing increasing pulmonary vascular resistance, pulmonary artery pressure and consecutive right heart failure. Moreover, pulmonary vascular remodeling is also found in hypoxic lung diseases and lung cancer, where it substantially contributes to disease related mortality.
PAH serves as our model diseases as it is characterized by initial microvessel loss through aberrant endothelial cell apoptosis in conjunction with neointima formation and adventital thickening by uncontrolled proliferation of smooth muscle (like) cells and fibroblasts.
All currently approved PAH therapies mainly focus on dilating the structurally abnormal vessels and but do not decisively reverse vascular remodeling.
Pathological remodeling of the pulmonary vasculature is closely related to (epi)genetic changes mediated by dysfunction of canonical and non-canonical Bone Morphogenetic Protein Type 2 Receptor (BMPR2) signaling via downstream transcription factors (TFs) . BMPR2 is dysfunctional in the PA of the majority of PAH patients, whether familial, idiopathic or associated with a variety of underlying medical conditions (APAH).
In healthy PA and lung microvascular endothelial cells (ECs) activation of non-canonical TF complexes downstream of BMPR2 is important to maintain mitochondrial structure and function, DNA integrity, angiogenetic plasticity, recovery from genotoxic & oxidative stress and lastly maintain EC survival in order to prevent pathological vascular remodeling.
In the pulmonary vasculature, the transcriptional landscape (as well as its interdependency) facilitating EC survival, maintaining DNA and vessel integrity is incompletely understood. Elucidation of the epigenetic and transcriptional regulatory mechanisms could help identify novel vasculoregenerative strategies to reverse detrimental pathological PA remodeling found in the lungs of transplanted PAH patients.
Scientific Approach
We use systems biology methods as well as genetic and functional approaches to study the molecular pathogensis of pulmonary vascular diseases.
By utilizing affinity purification and plasma proteomics, whole-genome TF binding analysis (ChIP-seq), whole-transcriptome analysis (RNA-seq), public data mining,
in silico co-occupation studies, immunohistochemistry, organo-specific genetic in vivo tools (lung EC-specific AAV), as well as molecular and functional cell biology methodologies, we aim